Alternatives to R134a
In automotive air conditioning systems with open compressors and hose connections in the refrigeration cycle, the risk of leakage is much higher than with stationary systems. Therefore, with a view to reducing direct emissions in this area of application, an EU Directive (2006/40 / EC) was adopted. Among other things, in the framework of new vehicle type approvals since 2011, only refrigerants with a GWP <150 have been approved. This eliminates the previously used in these systems R134a (GWP = 1430).
Meanwhile, alternative refrigerants and new technologies have been developed and tested. In this context, the use of R152a was further investigated. For some time, however, the automotive industry has agreed on system solutions with so-called "low GWP" refrigerants. The latter are discussed below.
R152a - an alternative to R134a (?)
R152a is very similar in terms of volumetric cooling capacity (about -5%), pressure levels (about -10%) and energy efficiency compared to R134a. Mass flow, vapor density and thus also the pressure drop are even cheaper (about -40%). R152a has been used as a component in blends for many years but not as a single-fluid refrigerant. Particularly advantageous is the extremely low global warming potential (GWP = 124).
However, R152a is flammable - due to the low fluorine content - and classified in safety group A2. This means increased safety requirements that require individual constructive solutions and safety measures as well as appropriate risk analyzes. For this reason, the use of R152a in vehicle air conditioning systems is unlikely.
"Low GWP" HFO refrigerant R1234yf and R1234ze (E)
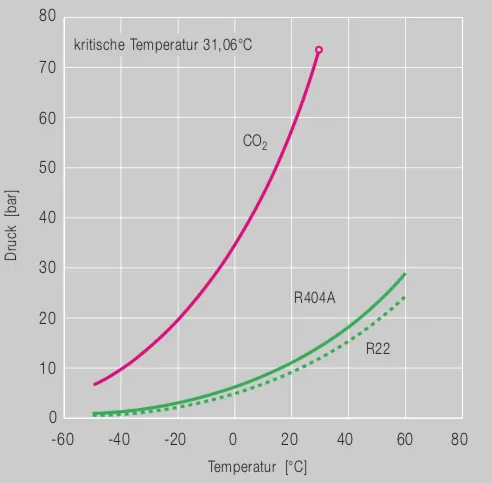
The ban on the use of R134a in automotive air conditioning systems within the EU has initiated a number of research projects. In addition to CO 2 technology (page 35), refrigerants with very low GWP values and similar thermodynamic properties as R134a have now been developed. At the beginning of 2006, two refrigerant blends were initially introduced under the names "Blend H" (Honeywell) and "DP-1" (DuPont). INEOS Fluor followed with another variant under the trade name AC-1. All refrigerants were, in the broadest sense, mixtures of different fluorinated molecules.
During the development and testing phase it became obvious that not all acceptance criteria could be met. Further investigations with these mixtures were therefore discontinued.
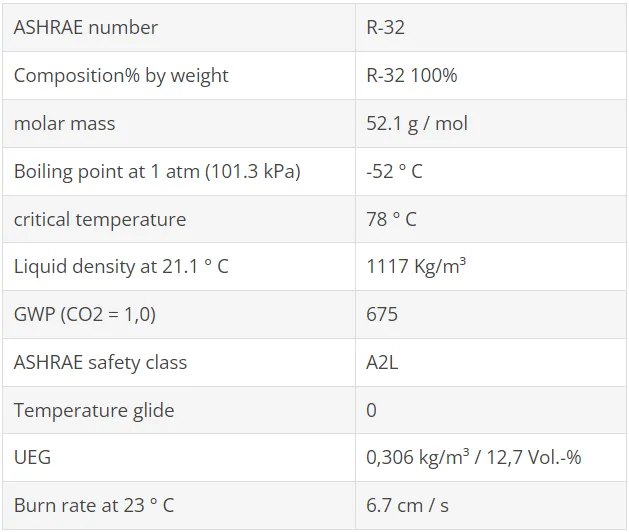
DuPont (now Chemours) and Honeywell combined their research and development activities in a joint venture focused on 2,3,3,3-tetrafluoropropene (CF3CF = CH2). This refrigerant, designated R1234yf, belongs to the group of hydro-fluoro-olefins (HFO). These are unsaturated HFCs with a chemical double bond.
The global warming potential is extremely low (GWP 100 = 4). Upon release into the atmosphere, the molecule breaks down rapidly within a few days, resulting in a very low GWP. However, this raises certain concerns about the long-term stability in the refrigeration cycle under real conditions. Extensive tests, however, have shown that the required stability for automotive air conditioning systems is met.
In the meantime, it has been determined that the potentially increased risk of flammability of the refrigerant in vehicle air conditioning systems can be avoided by appropriate design measures. However, there are also studies (eg by Daimler-Benz), in which an increased risk was detected. Various manufacturers have therefore intensified the development of alternative technologies again.
Toxicity studies show very positive results. The same applies to compatibility tests with the plastic and elastomeric materials used in the refrigeration cycle. Lubricants sometimes show an increased chemical reactivity, which can be suppressed by appropriate formulation and / or addition of "stabilizers".
The operating experience gained so far in laboratory and field tests give reason for a positive assessment, especially with regard to the performance and efficiency behavior. Cooling capacity and coefficient of performance (COP) are within the range of about 5% of the normal applications of automotive air conditioning in comparison to R134a. If the system is adapted accordingly, the same performance and efficiency can be achieved as with R134a.
Critical temperature and pressures are also similar, vapor densities and mass flow about 20% higher. The discharge gas temperature is up to 10 K lower in this application.
In view of the relatively simple conversion of automotive air conditioning systems, this technology has prevailed over the competing CO 2 systems.
However, as explained earlier, due to the flammability of R1234yf, further technical solutions are coming into focus.
These include active extinguishing equipment (eg with argon), but also the further development of CO 2 systems.
Further applications for HFO refrigerants
The use of R1234yf in other mobile climate applications is also considered, as well as in stationary air conditioning and heat pump systems. However, the fill level limits for A2L refrigerants (eg EN378) must be taken into account, which limit the use accordingly. There are also questions about the long-term stability in the refrigeration cycle in the usually very long life cycles of such systems.
For applications requiring operation of A1 (neither flammable nor toxic) refrigerants, R134a alternatives with lower GWP based on HFO / HFC blends have already been developed. They have been used in real facilities for some time.
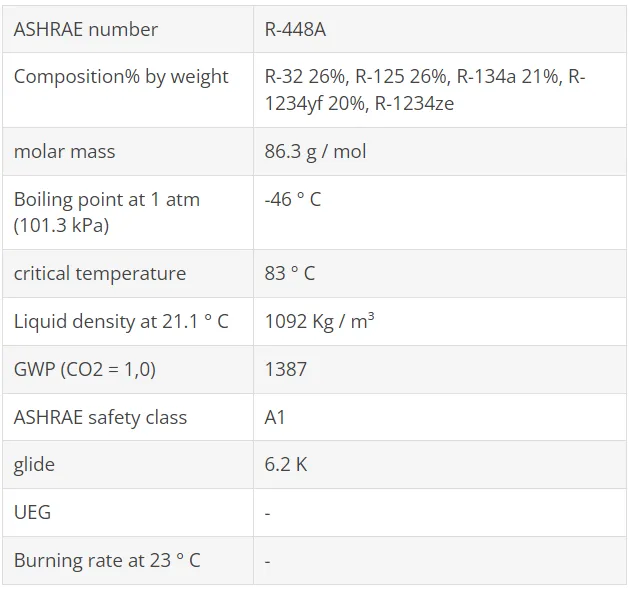
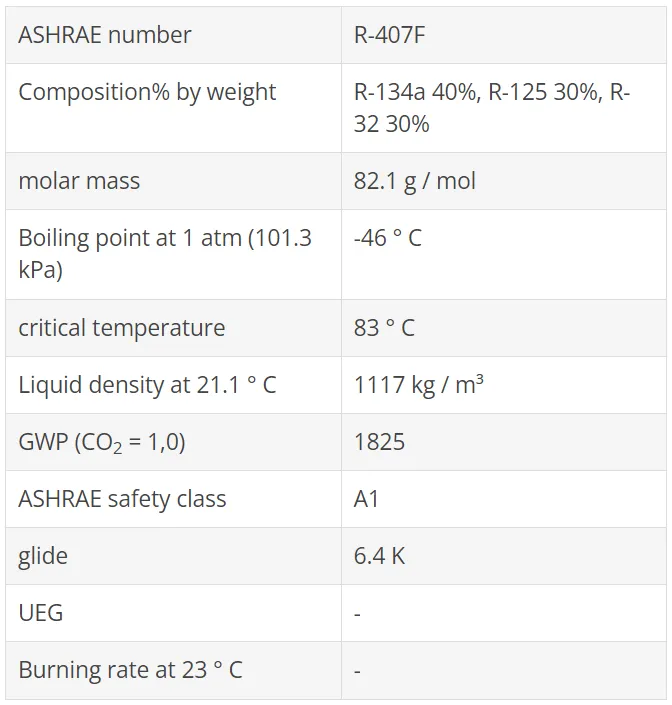
R1234yf, as well as the R1234ze (E) described below, are also used as basic components in HFO / HFC blends. These blends have been developed as "Low GWP" alternatives to R134a, R404A / R507A, R22 / R407C and R410A to meet regulatory requirements for reducing F-Gas emissions (eg EU F-Gases Regulation). Some of these refrigerants have already been tested in terms of refrigeration capacity and efficiency as part of AHRI's Alternative Refrigerants Evaluation Program (AREP), and have also been used in real facilities.
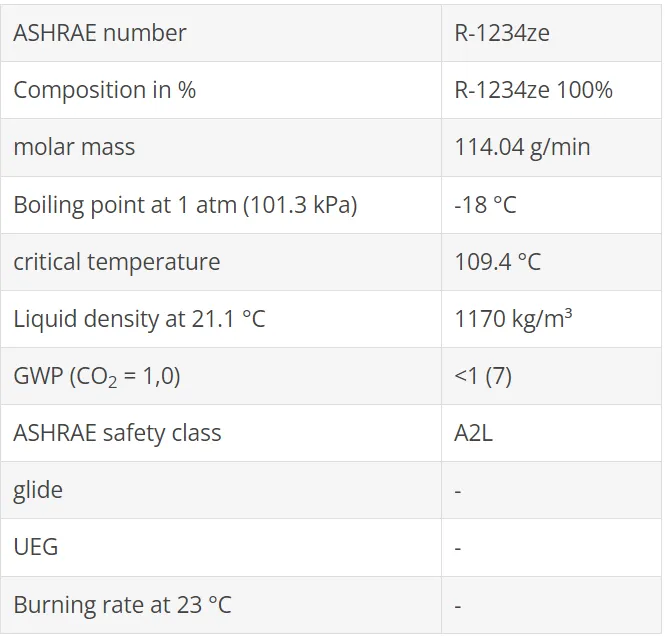
From the group of hydro-fluoro-olefins is another substance with the name R1234ze (E) is available, which has been used mainly as a propellant for PU foam and aerosol. R1234ze (E) differs from R1234yf by a different molecular structure. The thermodynamic properties also provide favorable conditions for use as a refrigerant. The global warming potential is also very low (GWP100 = 7).
There is some uncertainty about flammability. In safety data sheets, R1234ze (E) is declared non-flammable. However, this only applies to transport and storage. When used as a refrigerant, there is a higher reference temperature for flammability tests of 60 ° C. At this temperature, R1234ze (E) is flammable and therefore classified as R1234yf in safety group A2L.
R1234ze (E) is sometimes referred to as R134a substitute but is more than 20% lower in volumetric refrigeration capacity than R134a or R1234yf. The boiling point (-19 ° C) also limits the application at lower evaporation temperatures strong. The preferred use is therefore in liquid chillers and high temperature applications. For more information, see page 36, "Special applications".
With kind permission of Bitzer Kühlmaschinenbau GmbH
Source: Bitzer Refrigerant Report 19