SI - Units
SI-System
Internationally, they agreed on a uniform mass system, the SI system (Systeme International d'Unités). For some countries, the introduction of the SI system is an ongoing process. This document is based on the SI system. However, where it makes sense for traditional or other reasons, information is also given in the metric system or other common units. The following table lists the SI units and other common units for the state variables used in this document.
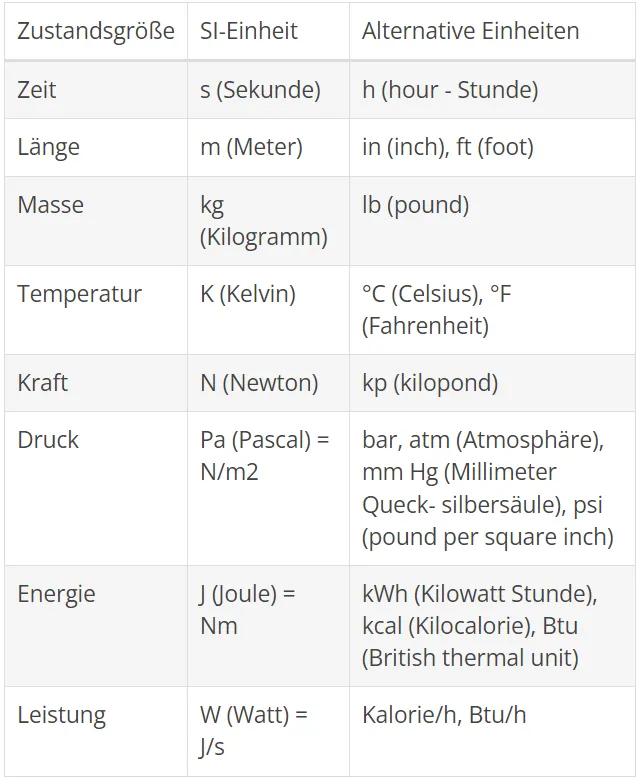
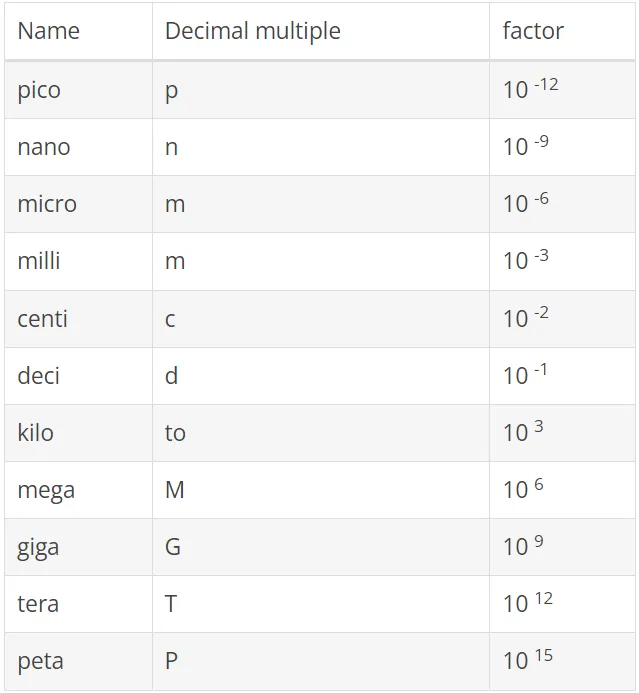
The practical application of the SI units is sustainably linked to the application of the decimal multiples and avoids either very short or long numbers. For a number of the most common decimal multiples, please refer to the table below. Example: The atmospheric pressure is 101325 Pa. At use of decimal multiples according to the following table, the best solution should be written 101,325 kPa. The choice of multiples is "free", but the best choice will normally be to give a value in a range between 0.1 and 999.9. Multiples can not be used for combined SI units - except when using [kg]. Example: 2000 W / m 2 K and not as 2 kW / m 2 K.
Temperature
Temperature is a key feature in refrigeration. Almost all refrigerant systems have the goal of reducing the temperature of an object, such as the air stored in a room or in a room.
In refrigerant systems, the temperature is given in degrees Celsius [° C]. Celsius is not an absolute temperature scale, but the reference point (0 ° C) is defined by the freezing point of water.
The SI unit for temperature in Kelvin [K] is an absolute temperature, since the reference point [0 K] is the lowest temperature that could be achieved in theory. The only difference between Kelvin and ° Celsius is the reference point, ie that a temperature difference of 1 ° C corresponds exactly to the temperature difference of 1 K. Scientifically, refrigeration technology describes temperature differences in [K] instead of [° C]. This practice reduces possible confusion of temperatures and temperature differences.
Power and pressure
The SI unit for force is Newton (N), which currently corresponds to one [kg m / s 2 ]. If a force acts on a surface, its influence depends on the size of that surface. A convincing example of this is the fact that you sink less deeply on a snowpack with skis than without. Namely, they distribute the weight over a large area, so that the weight per unit area becomes relatively small. Pressure is defined as the relationship between force and surface on which it acts. In the example with the skis, the force (gravity) is the same in both cases, only the area is different. Without skis, the area is small and the pressure is high, with skis, the area is large and the pressure is small.
In refrigeration, pressure is usually associated with fluids used as refrigerants. When a substance in liquid or gaseous form is stored in a closed container, the gas exerts a pressure on the inner walls of the container. The pressure of the gas on the inner surface divided by its area is called absolute pressure. For practical reasons, the value of the pressure is given now and then as "pressure above atmospheric pressure" - means the atmospheric pressure (1013 5 kPa = 1.013 bar) is subtracted from the absolute pressure. The pressure above atmospheric pressure is often referred to as gauge pressure.
The unit used should reflect whether absolute pressure or gauge pressure is given. An absolute pressure is indicated by using a lowercase letter "a" and a gauge pressure is indicated by a lowercase letter "g".
Example: The absolute pressure is 10 bar (a), which is converted to a manometer pressure of (10 - 1.013) bar (g) ≈ 9 bar (g). This combination of SI units for pressure in [Pa] is not recommended.
Other common units for pressure are mm of mercury [mmHg] and meter of water head [mwg]. The latter is often used in conjunction with pumps, as an indicator of the height of the water column that the pump can generate. Vacuum is defined as an absolute pressure of 0 Pa - but since it is nearly impossible to achieve the term "vacuum", it is common practice to describe pressure much lower than atmospheric pressure. Example: The absolute pressure is 0.1 bar (a), which is converted to a pressure gauge pressure of (0.1 - 1.013) bar (g) ≈ -0.9 bar (g). Vacuum is also often given in Torr (1 Torr = 133.3 Pa) or in millibars (one thousandth of a bar).
Warmth, work, energy and performance
Heat and work are forms of energy, ie they can be transferred between objects or systems. The transfer of heat is closely related to the temperature (or temperature difference) that exists between two or more items. As such, heat is always transferred from a higher temperature object to a lower temperature object. Heating a pot of water on a hotplate is a fitting, everyday example of heat transfer. The hotplate gets hot and heat is transferred to the water via the bottom of the pan. The heat transfer to the water causes a temperature increase of the water. In other words, heating an object is the same as transferring energy (heat) to the object.
In many practical applications, it is necessary to reduce the temperature of an object rather than raising the temperature. Following the above example, this can only be achieved with an object that has a lower temperature than the object to be cooled. Bringing these two objects in contact, a heat transfer is caused away from the object to be cooled, consequently, the temperature drops. In other words, cooling an object is the same as transferring energy (heat) away from the object.
The typical transfer of work is via a mechanical shaft rotating in an electric motor or in an internal combustion engine. Other forms of work transfer are possible, but a rotating shaft is the most common method used in refrigerant systems.
As mentioned, both heat and work are forms of energy. The methods of transfer between objects are different, but in a process involving transfer of heat and labor, it is the sum of transfer of heat and labor that determines the outcome of the process.
The SI unit Joule [J] is used to measure energy, heat and work. The amount of energy needed to heat 1 kg of water from 15 ° C to 16 ° C is 4.187 kJ. These 4.178 kJ can be transferred as heat or work - but heat will be the most common and suitable solution in this process. Different substances require different amounts of heat to increase their temperature by 1 K: 1 kg of iron requires 0.447 kJ, while 1 kg of air requires about 1 kJ.
The "specific heat" of a substance is the amount of heat with which 1kg can be heated by 1K. It is tabulated for a long list of substances and substances and has the SI unit J / kg K. The measure by which energy is transmitted is called power. The SI unit for power is Watts (W).
Example: If 10 J are transmitted per second, the rate of energy transfer is 10 J / s = 10 W. In the SI system the selection of the unit for power is the same as for transfer of heat and work. In other system units, the transmission of heat and work has different units.
State changes
All substances can occur in three aggregate states: solid, liquid or gaseous. The best known example is water: when solid, it appears as ice, in gaseous form as vapor, while we encounter it in its liquid form throughout our lives. For water, the three phases have different names - which makes it somewhat difficult to consider it as a model substance. The solid form is called ice, the liquid form is simply called water, and the gaseous form is called water vapor. All these states have one thing in common: the water molecule appears in an unchangeable form; ie that ice, water and water vapor can be named with the same molecular name: H2O. When a substance passes from the solid phase to the liquid phase, This process is called melting (liquefying) and when it goes further into the gaseous phase, it is called boiling (evaporation). Is it in the opposite direction? a gaseous substance enters the liquid phase, which is called condense, and when it goes further into the solid phase, the process is called freezing (solidifying).
At constant pressure, the transition process has a significant property. When ice is heated at 1bar, its temperature rises until reaching 0 ° C - then the ice begins to melt. During the melting process, the temperature does not change - all the energy that is transferred into the ice / water mixture becomes used to melt the ice and not to heat the water. Only when the ice has completely melted, the further energy transfer will cause a temperature increase.
The same type of reaction can be observed when water is heated in an open pot. The water temperature rises until it reaches 100 ° C, then the evaporation begins. During the evaporation process the temperature remains at 100 ° C. Only when all liquid has evaporated, the temperature of the remaining water vapor rises.
Temperature and pressure determine whether a substance is in a solid, liquid or gaseous state - or in two or all three states. In our environment iron comes in its solid state, water solid, liquid and gaseous, and air in its gaseous state.
Different substances have different melting and boiling points; For example, gold melts at 1064 ° C, chocolate at 26 ° C and most refrigerants melt at temperatures around -100 ° C. Whether a substance occurs in two of its phases at the same time - or undergoes a phase change - is dependent on pressure and temperature. When the two phases occur in a closed vessel and both phases are in a thermal steady state, the state is considered saturated. As the temperature of the two-phase mixture increases, so does the pressure in the vessel. The relationship between pressure and temperature for the saturated state (liquid and vapor) is commonly referred to as a vapor pressure curve. By means of steam-pressure-curve one determines the pressure with evaporation or liquefaction.
Latent heat
It is important to note, in the process of ice melting, that the amount of energy that needs to be transferred to melt 1 kg of ice is much higher than the energy required to increase the temperature of 1 kg of ice, eg 1K. In section .4 the specific amount of heat for water was given as 4.187 kJ / kg K. The energy to melt 1 kg of ice is 335 kJ. The same amount of energy, which melts 1 kg of ice, can raise the temperature of 1 kg of water to (335 kJ / 4.187 kJ / kg K) = 80 K!
Back to the process of water boiling, here the energy needed to evaporate 1 kg of water would be 501 kJ. The same amount of energy that evaporates 1 kg of water can not heat the temperature from 1 kg, but from 6 kg to 100 ° C!
These examples show that energy transfer relative to the transition processes between the phases is important. For this reason, ice is used for cooling, it takes a large amount of energy to melt the ice, and during this time the temperature remains at 0 ° C. The refrigeration effect in refrigerant systems is based on the controlled application of the phase change in the evaporation process. When the refrigerant evaporates, it absorbs energy (heat) from its environment. By thermal contact of an object with the evaporating refrigerant, it is cooled.
Overheating
Overheating is an important term in the vocabulary of refrigeration - but unfortunately it is used differently. It may describe a process in which refrigerant vapor in the saturated state is heated to a higher temperature. It can also be used to describe the end of the process described above.
Overheating can be explained as a temperature difference - between the temperature measured with a thermometer and the saturation temperature of the refrigerant measured with a pressure gauge. For this reason, the overheating can not be determined by a simple temperature measurement alone - a measurement of the pressure or the saturation temperature is also required.
If an overheating is numerically determined, it must be given as a temperature difference, and consequently with the unit [K]. If specified in [° C], the cause of the fault may be where the temperature for the overheating indication was measured or vice versa.
The evaporation process of a refrigerant system is one of the processes where the term overheating is used. This will be explained in more detail below.
Refrigeration diagrams
The characteristics of a refrigerant can be explained in a diagram, by means of which the abscissa and ordinate the relevant properties are explained. The most important properties for refrigerant systems are usually energy content and pressure. The thermodynamic property "specific enthalpy" stands for the energy content - assessed by the change of the energy content per unit of measurement of the refrigerant exposed in a process of a refrigerant system.
A diagram example based on the specific enthalpy (abscissa) and the pressure (ordinate) can be seen below. The pressure range typically applicable for a refrigerant is large - therefore, graphs require a logarithmic scale for the pressure.
The diagram is arranged so that it shows liquid, steam and the mixing range of the refrigerant. Liquid can be found on the left (with a low energy content) and steam on the right (with a high energy content). Between the two is the mixing area. The areas are limited by a line - the so-called saturation line. The essential processes of evaporation and liquefaction are explained.
The idea of using such a refrigeration diagram is to represent the process in a refrigeration system in a way that makes analysis and evaluation of the process easier. When using a diagram, system operating conditions (temperature and pressure) are to be determined in a relatively simple and fast manner. Charts are still used to analyze a refrigeration process. However, a number of PC selection programs are now available that can perform the same analysis faster and with more detail.








