ODP - Ozone depletion potential of refrigerant
Definition
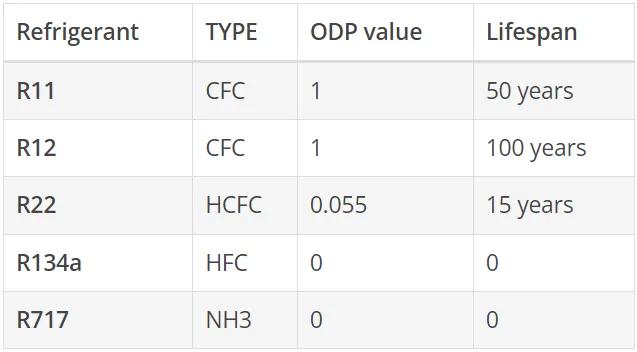
ODP - Ozone Depletion Potential - (ozone depletion potential) is a unitless value and allows the representation of the environmental damage potential of refrigerants in relation to a reference substance. The reference substance is the refrigerant R11 with the ODP value of 1 (100%).
Degradation of the ozone layer
In 1974, the chemists Frank Sherwood Rowland and Mario Molina publish the hypothesis that chlorine in the earth's atmosphere is responsible for the destruction of ozone. CFCs, the abbreviation for chlorofluorocarbons. Thirty years ago, this gas was used in large quantities, for example, as a propellant for spray cans, as a solvent, as a refrigerant and for plastic foaming. The substances remain in the atmosphere for decades and sometimes centuries, where they deplete the ozone layer. Other substances, such as certain fire extinguishing agents (halons), reduce the ozone layer. In addition, CFCs and halons also have a high global warming potential ( GWP) and thus contribute to global warming. Due to the long life of the CFCs, these can be distributed throughout the atmosphere and rise after about 7 - 10 years in the ozone layer. Here only CFCs are split by high-energy solar radiation. The released chlorine immediately reacts with ozone. In addition, a chlorine atom can destroy up to 100,000 ozone molecules because it acts catalytically.
The ozone depletion is therefore a chemical reaction, which also takes place only in low temperatures of about -60 to - 70 ° C. The condition we find mainly on the earth's poles such as the Antarctic. The reason for the so-called "ozone hole".
On 16 September 1987, 24 states and the European Community sign the Montreal Protocol. The Contracting Parties undertake to take concrete steps to reduce the production and use of ozone-depleting substances.
The Montreal Protocol enters into force on 1 January 1989.
All 197 UN states have joined the protocol.
Conclusion
The Montreal Protocol has called for a reduction and phasing out of the use of CFC-containing substances. The rapid exit of the acceding countries had to look for the positive side effect of alternative solutions. Because the HFCs, which were later heavily marketed as alternatives, were not yet available, in some applications halogen-free (natural) substances were established, which were not displaced again.
Köpenicker Straße 325
Haus 11
12555 Berlin
Germany




